TEF-Health as a whole
To make the EU the place where AI excellence thrives from the lab to the market. World-class Testing and Experimentation Facilities (TEFs) for AI.
TEF as a whole
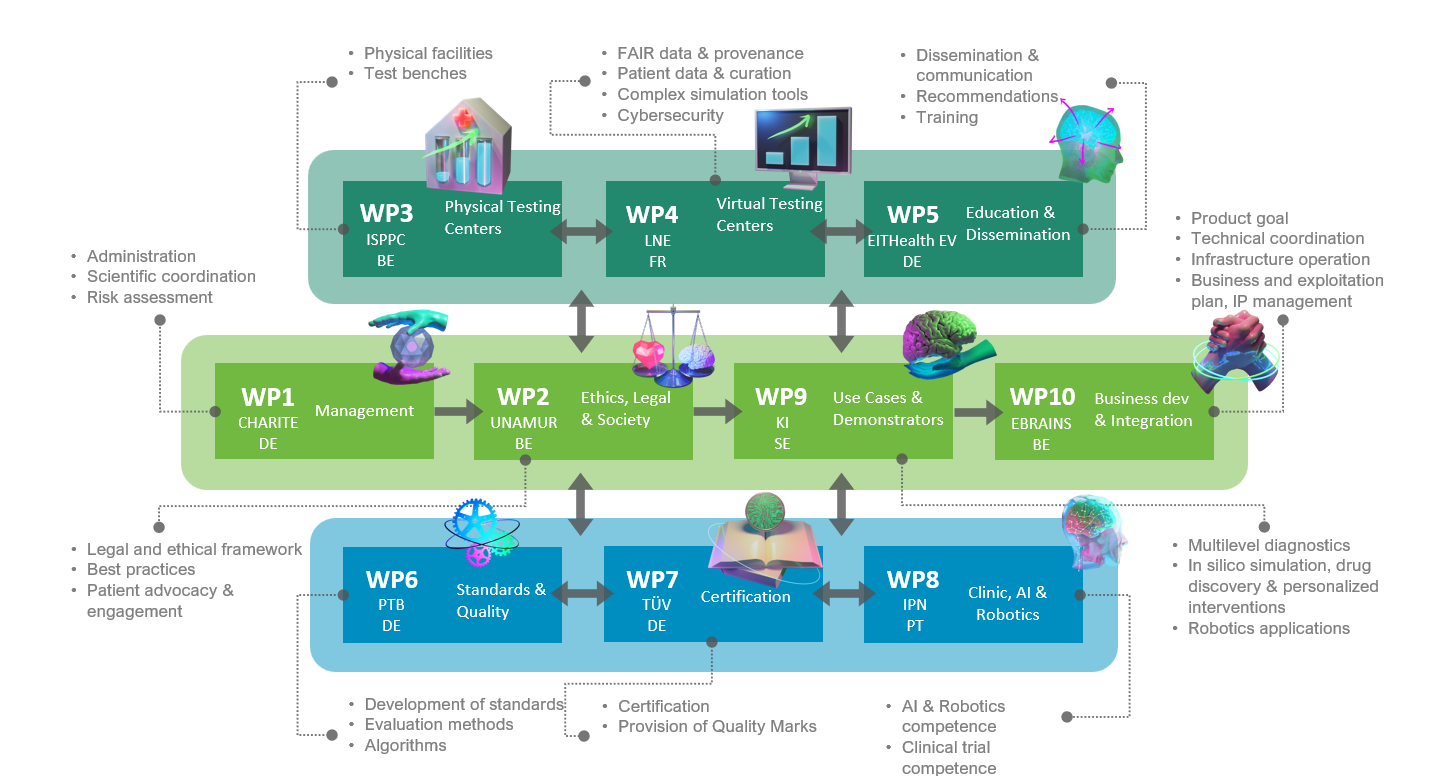
Technological advances in the field of AI and robotics are being made at a breathtaking pace – and the healthcare sector is not spared from these developments. Yet it goes without saying that new medical devices and procedures must first prove their safety and usefulness before they can be adopted in clinical practice. In the European Union, the areas of AI and robotics, which are set to have a far-reaching impact on the healthcare sector, especially have to meet high quality requirements, but there is still a lack of testing infrastructure for developing standards, validating innovations, and certifying new products.
This is precisely where the Testing and Experimentation Facility for Health AI and Robotics (TEF-Health) comes in. The new project, supported by the EC and national funding agencies with a total of about €60 million, aims to “facilitate and accelerate the validation and certification of AI and robotics in medical devices,” explains Prof. Ritter, who coordinates the consortium and heads the Brain Simulation Section at the BIH. In total, 51 academic and private partners from nine European countries are involved in the project, integrating existing infrastructures as well as building new ones. The project started on 1 January 2023.
“With TEF-Health we mainly want to test novel AI approaches in real-world scenarios,” says Prof. Ritter. This includes new software used in areas such as patient care and diagnostics, as well as devices controlled by artificially intelligent programs, some of which are designed for direct use on humans – such as surgical and nursing robots. “We will evaluate how to facilitate market access and acceptance of these smart technologies,” Prof. Ritter reports.
Project partners plan to develop new regulatory and ethical requirements, including, for instance, standardized testing protocols and certifications or a specific code of conduct for use of the technology. In addition, the necessary technical and administrative procedures must be developed and created. Also on board the TEF-Health project are therefore leading hospitals, universities, and clinical research institutions such as Sweden’s Karolinska Institutet, as well as state-designated testing organizations such as German safety inspector TÜV and the National Metrology Institute of Germany and its French counterpart, the “Laboratoire national de métrologie et d'essais” (LNE). The newly created evaluation resources and infrastructure will be made available to industry in the future in the form of fee-based services. “Widespread use of these comprehensive testing and evaluation tools will not only accelerate market access for innovative AI and robotics technologies, but will also ultimately boost public confidence in these new developments,” explains Prof. Ritter.
The TEF-Health project has the express aim of generating and consolidating sustainable collaborations between industry, academic research, and other players. “Long-term partnerships in innovation networks have shown to provide a particularly favorable environment for the transfer of technology from research to practice,” Prof. Ritter explains. “What’s more, close cooperation between the various partners avoids duplicating work already done.” This, she says, makes sure investments are used for highest impact. The close collaboration will also help to ensure that going forward research findings are translated more quickly into new products and services. Ultimately, the entire value chain in AI and robotics technologies for healthcare will benefit from this – which in turn will “increase the prosperity and quality of life of society as a whole,” predicts Prof. Ritter.
So in the end, TEF-Health will contribute to Digital Europe’s overall aim of increasing the effectiveness, resilience, and sustainability of European health and care systems and reducing healthcare delivery inequalities in Europe, while ensuring compliance with relevant legal, ethical, quality, and interoperability standards and requirements.
What is TEF?
TEFs are specialised large-scale reference sites open to all technology providers across Europe to test and experiment at scale state-of-the art AI solutions, including both soft-and hardware products and services, e.g. robots, in real-world environments.
These large-scale reference testing and experimentation facilities will offer a combination of physical and virtual facilities, in which technology providers can get support to test their latest AI-based soft-/hardware technologies in real-world environments. This will include support for full integration, testing and experimentation of latest AI-based technologies to solve issues/improve solutions in a given application sector, including validation and demonstration.
Four TEFs projects started on January 1st 2023. They focus on the following high-impact sectors:
Healthcare: project TEF-Health
Agri-Food: project agrifoodTEF
Manufacturing: project AI-MATTERS
Smart Cities & Communities: project Citcom.AI
Coordination
Prof. Dr. Petra Ritter
|
TEF-Health Coordinator |
Partners

Nodes


Germany | Berlin
CHARITÉ, BPWT, VdTÜV, KI Park
Germany | Braunschweig
PBT
Germany | Erlangen
FAU
Germany | Heidelberg
EIT H SI GmbH
Germany | München
TUM, Fraunhofer, EIT Health EV
France | Paris
LNE, CEA, EIT Health FR, HDH
France | Grenoble
CHUGA, UGA
France | Lyon
HCL
France | Rennes
CHU RENNES
France | Strasbourg
IHUS
Portugal | Coimbra
IPN, CHUC EPE
Portugal | Lisboa
SPMS, InnoStars
Portugal | Porto
CHSI, HCP
Slovakia | Martin
UHM
Slovakia | Bratislava
UK BA, STUBA
Slovakia | Zilina
UNIZA
Belgium | Charleroi
Humani, CETIC, BIOWIN
Belgium | Liège
WSL
Belgium | Mol
VITO
Belgium | Mons
MULTITEL
Belgium | Namur
UNamur, POLEM
Netherlands | Rotterdam
EIT Health BeNe
Italy | Roma
ISS
Italy | Genova
IGG, IIT
Italy | Lecce
INNOVAAL
Italy | Milano
POLIMI
Italy | Pavia
UNIPV
Italy | Povo
FBK
Czechia | Brno
MU
Finland | Helsinki
HUS, METROPOLIA, HELSINGFORS
PAN-EU | Brussels
EBRAINS
Sweden | Boras
RISE
Sweden | Stockholm
KI
Germany
| Berlin |
CHARITE BPWT VdTÜV KI PARK e.V. |
| Braunschweig | PTB |
| Erlangen | FAU |
| München | TUM FRAUNHOFER EIT HEALTH EIT HEALTH SI GmbH InnoStars |
Prof. Dr. Petra Ritter
Petra.ritter@charite.de
France
|
Paris |
LNE CEA Health Data Hub |
|
Grenoble |
CHUGA UGA |
| Lyon | HCL |
| Rennes | CHU RENNES |
| Strasbourg | IHU STRASBOURG |
Guillaume Bernard
Guillaume.Bernard@lne.fr
Slovakia
| Martin | Hospital Martin |
| Bratislava | UK BA STUBA |
| Zilina | UNIZA |
Miroslava Fövényes
miroslava.fovenyes@unm.sk
Belgium
| Mons | MULTITEL |
| Liège | POLE MECATECH WSL |
| Mol | VITO |
| Charleroi | BIOWIN Humani CETIC |
| Namur | UNamur |
| Rotterdam | EIT Health BeNe |
Anne-Laude Cadji
cadji@multitel.be
Advisory Board
Alexandra von Korff |
 |
Luis Marti-BonmatiScientific coordinator EUropean Federation for CAncer IMages EUCAIM |
|
Remke BurieUniversity of Twente, Managing Director Technical Medical Centre |
|
Birgit Bauer |
|
Thomas Schulthess |
 |
Roadmap
Roadmap
- Q1 2023
- Q3 2023
- Q1 2024
- Q4 2024
- Q4 2025
- Q1 2026
- Q3 2026
- Q1 2027
- Q3 2027
- Q4 2027
- >2028
Market analysis
Our market research aims at identifying Use Case domains for TEF-Health with least financial obstacles / lack of market
finance. While not being exclusive to other domains the focus of TEF-Health in terms of
Use Case selection are:
Neurotec, Cancer, CardioVascular, Intensive Care and Neurotec.
TEF-Health has assessed how the different domains are positioned in relation to emerging trends in the area. In the preliminary market analysis for TEF-Health we have used several methods, including contextual analysis, drivers’ assessment, SWOTs, Horizon scanning, Trends analysis, Risks analysis, road mapping. With the selected Use Cases TEF-Health ensures that we address a wide range of users and hence maximise impact.
Data Management Plan
Data Management Plans (DMPs) are a key element of good data management. A DMP describes the data management life cycle for the data to be collected, processed and/or generated within a project.
As part of making research data findable, accessible, interoperable and re-usable (FAIR). A DMP typically includes information on:
- the handling of research data during & after the end of the project
- what data will be collected, processed and/or generated
- which methodology & standards will be applied
- whether data will be shared/made open access and
- how data will be curated & preserved; including after the end of the project.
Virtual testing services catalog vs1
We will set up virtual testing and experimentation facilities and services AI in healthcare. Subobjectives are:
-set up the evaluation protocols, tools and digital facilities to provide virtual testing services based on datasets
-set up the evaluation protocols, tools and digital facilities to provide virtual testing services based on simulator and mixed testing environments (coupling simulators and physical test benches).
-set up the evaluation protocols, tools and digital facilities to provide cybersecurity testing services.
-set up a cross European digital testing platform and the TEF dataspace that will allow access to certain services and databases remotely.
Catalogue of testing centers
A catalogue on physical and virtual testing centers will be available to interested endusers.
The real testing facilities (hospital platforms, physical infrastructures, living labs, etc.) and the laboratory testing ones.
We will carry out tests and experiments of AI solutions (software and hardware) in large-scale and sustainable real or realistic environments.
Provide access to clinical settings is crucial, and not only to conduct clinical trials and use clinical datasets of infrastructures, but also to get feedbacks from healthcare professionals and patients.
In addition, we will set up virtual testing and experimentation facilities and services AI in healthcare.
Catalogue of quality criteria and reference metrics
We will define quality criteria and reference metrics
- to evaluate data for AI
- to evaluate AI algorithms
-to establish standards for agile certification process.
Price lists
To comply with state aid under GBER, the TEF draws up a price list (differentiated per node) for the services offered, based on market prices
if these exist, and display the available reductions it plans to offer to SMEs.
Larger companies must pay the market prices according to the TEF’s price list.
While an initial catalogue of services with price lists and reductions for SMEs has been developed,
TEF-Health commits to further advance its methodology on deriving its price lists.
Roadmap of the virtual testing facilities
We will set up a setting up virtual testing and experimentation facilities and services AI in healthcare.
These facilities and services will be based on datasets and/or simulators, cybersecurity. Furthermore we aim at setting up a digital testing platform to make several virtual testing services remotely accessible, when conditions allow (non-confidential data, confidential algorithms, etc.).
Full compliance with the ethical requirements will be ensured.
Exemplary agile certification compliant with MDR
We will develop reference process for ‘agile certification’ of medical devices according to MDR.
TEF-Health will
-establish links to notified bodies regarding procedure, touchpoints, and possible legal requirements/changes for an agile certification approach.
-use a small number of use cases based on existing / soon to be launched products, conduct exemplary certifications to prove out / improve the agile certification.
Demonstrator cases
By interactively evaluating potential use cases, we will promote TEF-SME interaction and guide the TEF in selecting representative and strategically suitable internally and externally requested demonstrator cases.
A demonstrator case might e.g.
- use an already certified AI product, execute agile certification,
- evaluate benefits of agile over standard certification,
- use results to fine-tuneagile certification,
- apply refined process to certify a new AI product.
Standards for AI in medical devices
TEF-Health aims to establish an agile certification process for AI in medical devices which aims to reduce time-to-market for these products, promote innovation in AI in health care by SMEs while maintaining high quality standards that make AI in medical devices trustworthy.
Q&A
The Concept of Testing and Experimentation Facilities
read more about e.g.
What is a TEF?
Who can use the TEF?
Will TEFs collect or harvest data?







